The FDA approved the first new drug to treat Alzheimer’s disease in 18 years on Monday, as the number of San Diegans living with dementia hits a record high.
The new drug, called aducanumab, will be an option for people in the early stages of memory loss. This is the first treatment approved for Alzheimer’s disease since 2003.
Here are some important things to know about the drug, also known as Aduhelm:
- This drug was developed for people living with mild cognitive impairment or in the early stages of Alzheimer’s disease.
- It is the first treatment determined to slow progression of the disease by reducing amyloid beta plaques in the brain – not just address symptoms.
- Experts have been split on its effectiveness, and there will still need to be a post-approval trial to verify its clinical benefit. If it fails, the FDA can remove it from the market. Read the FDA decision by clicking here >>
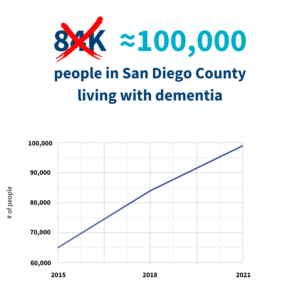
In other big news – according to a new report, the number of San Diegans living with dementia has increased to ≈ 100,000. In 2018, that number was 84,000. In 2015, it was 65,000. This is a sobering jump, and a reminder that dementia is very much a local health crisis. Alzheimer’s San Diego President/CEO Eugenia Welch spoke with CBS News 8 about the local numbers, and the approval of aducanumab. You can watch the video below:
At Alzheimer’s San Diego, we hope to one day close our doors because a cure has been found. But until that day comes, it is an honor to serve you. Please give us a call for free support at 858.492.4400. You are not alone.